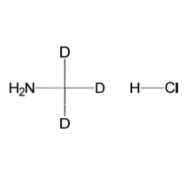
Methyl D3 Amine Hydrochloride
Molecular Weight: CD3NH2 · HClMolecular Formula: 49.00 g/mol
CAS Number: 7436-22-8
Methyl D3 Amine Hydrochloride, also known as deuterated methylamine hydrochloride, is a captivating chemical compound that has been garnering significant attention in scientific circles. Its unique isotopic labeling and versatile applications have made it an invaluable tool in various fields of study. Here, we aim to provide you with an in-depth exploration of Methyl D3 Amine Hydrochloride, uncovering its properties, uses, and the latest advancements in research. Join us on this scientific journey as we delve into the wonders of Methyl D3 Amine Hydrochloride and its remarkable potential in shaping the landscape of chemistry.
What are the uses of Methyl D3 Amine Hydrochloride ?
Methyl D3 Amine Hydrochloride, a deuterated form of methylamine hydrochloride with three deuterium atoms, has diverse and valuable applications in both scientific research and industrial settings. One of its primary uses is in drug metabolism studies. As a labeled compound, Methyl D3 Amine Hydrochloride is instrumental in investigating the metabolic pathways and biotransformation of pharmaceutical agents. By incorporating the deuterated methylamine into drug candidates, researchers gain crucial insights into their metabolism and potential drug-drug interactions, contributing to the development of safer and more effective medications.
Another significant application of Methyl D3 Amine Hydrochloride is in isotope labeling studies. The presence of deuterium atoms in the methyl group allows scientists to use it as a tracer and marker in various chemical reactions. Researchers can track the fate and transformation of methyl groups in organic synthesis, environmental studies, and other chemical processes. The deuterium labeling provides a unique and distinct signal in analytical techniques, making it an essential tool for investigating reaction mechanisms and kinetics.
Moreover, Methyl D3 Amine Hydrochloride is used in the production of labeled compounds and intermediates for research purposes. Incorporating the deuterated methylamine into molecules of interest facilitates studies in fields like drug discovery, materials science, and catalysis. The presence of deuterium atoms enables researchers to track the behavior and transformations of these compounds, providing valuable insights into reaction pathways and facilitating the design of novel and more efficient processes.
Synonyms
- Deuterated methylamine hydrochloride
- Methylamine D3 hydrochloride
- Methyl-D3-amine HCl
- D3-labeled methylamine hydrochloride
- Perdeuteriomethylamine hydrochloride
Chemical Properties
- Molecular Formula: CD3NH2 · HCl
- Molecular Weight: 49.00 g/mol
- Appearance: White to off-white crystalline powder
- Solubility: Soluble in water, alcohol, and other polar solvents
- Molecular Formula: CD3NH2 · HCl
- Molecular Weight: 49.00 g/mol
- Appearance: White to off-white crystalline powder
- Solubility: Soluble in water, alcohol, and other polar solvents

